Tungsten: Properties, Production, and Applications
More than 350 years ago, it was observed that Chinese porcelain makers were adding to their designs a unique peach colour that wasn’t known or recognised in the rest of the world [1]. That remained until 1779 when the Irish chemist and mineralogist Peter Woulfe identified the existence of a new metal in samples of Sweden’s wolframite, but he did not separate it [2]. Later in 1781, German chemist Wilhelm Scheele continued the research and succeeded in isolating an acidic white oxide, which he deduced was the oxide of this new metal. The honour of discovering this metal was given to the Spanish chemists and mineralogists Juan Jose and Fausto Elhuyar, who in 1793 produced the same acidic metal but unlike any others, were successful in isolating the metallic tungsten [1].
Tungsten is a transition metal known for its outstanding properties, especially when combined with other elements in compounds such as tungsten carbide. The name Tungsten was derived from the Nordic words “tung" and "sten”, which combined mean “heavy stone” [3]. Tungsten is also known as wolfram, from which it takes its chemical symbol, W. The name wolfram was originated from the wolframite mineral.
The content of tungsten in the Earth’s crust is around 0.007% (global reserve 3.5 million tons). Today, China accounts for a significant majority of the global tungsten reserves [1]. Tungsten and its compounds have a wide spectrum of applications due to their physical and chemical properties.
Here, you will learn about:
The properties of tungsten
The sources of tungsten
The products and uses of tungsten
Future applications of tungsten
Properties of tungsten
Tungsten is a light grey or silvery-white metal that constitutes one of the toughest metals can be found in nature. The density of tungsten is significantly high, in fact, among the highest of all metals [2][4]. The table below shows some of the key features of each of tungsten and tungsten carbide. Notice how the addition of carbon increased its hardness significantly but lowered its melting point and thermal conductivity.
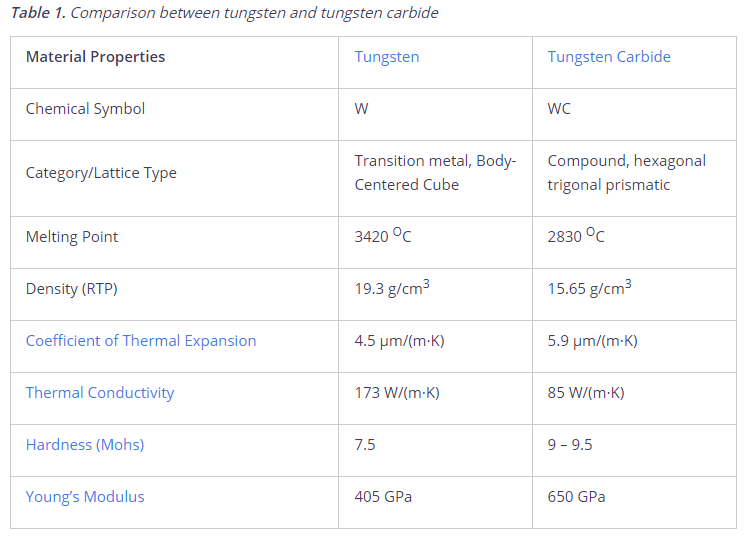
Tungsten has the highest melting and boiling points among all discovered elements and the lowest coefficient of thermal expansion (CTE) among metals.
Here's a property comparison from Matmatch that shows tungsten's ranking among other metals in terms of melting point, coefficient of thermal expansion, and density. Notice how tungsten ranks highest in melting point, lowest in CTE, and among the highest in density. Click on the figure or the link in the caption below to perform your own comparison scheme.
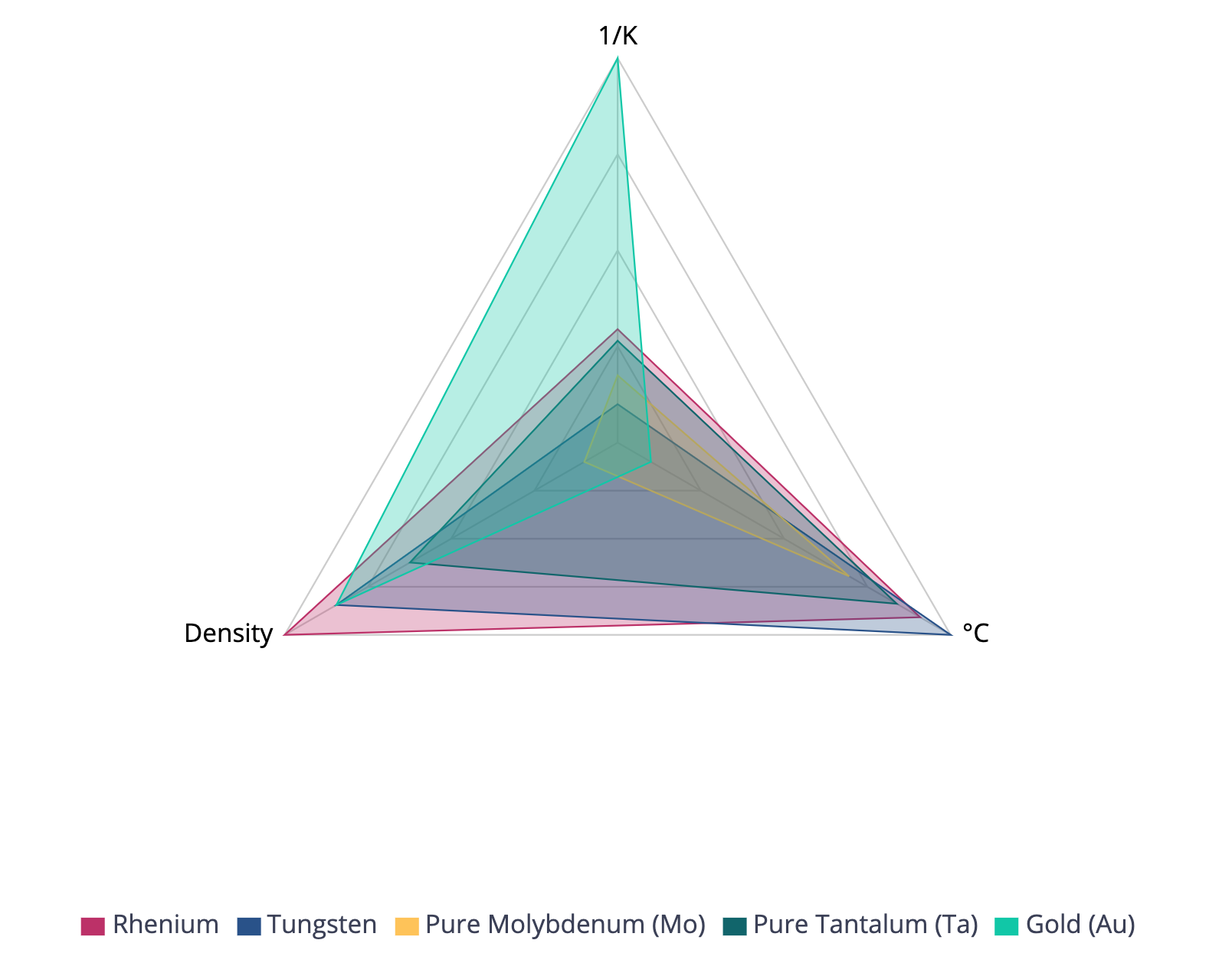
Figure 1. Property comparison between Tungsten and other metals showing their rankings for melting point, CTE, and density
This rare metal’s properties, whether in its pure or alloyed form, render tungsten quite useful for many modern-day applications. Tungsten rings, for example, are considered to be scratch resistant and unbreakable due to their incredible hardness and strength. The fact that they do not easily rust and are corrosion resistant to a high degree makes them even more alluring to the future of materials science.
